
Assistant Professor of the Institute for Tissue Engineering and Regenerative Medicine
Office: (852) 3943 9797
Email: anna.blocki@cuhk.edu.hk
Address: Room 425C, 4/F, Lo Kwee-Seong Integrated Biomedical Science Building, Area 39, CUHK
ORCID: 0000-0002-9878-3781
SBS Website: https://www2.sbs.cuhk.edu.hk/en-gb/people/academic-staff/prof-blocki-anna-maria
Personal Statement and Research Goals
The tissue microenvironment conveys information to regulate numerous processes critical for the functioning of the entire body down to responses of individual cells. The overall goal of Prof. Blocki’s research is to tap into nature’s wisdom to learn to decipher and engineer microenvironments that drive specific tissue processes including tissue healing and regeneration.
Engineering of bio-instructive matrices in vitro
The ECM is a biomaterial designed by nature, which has undergone more than 500 million years of material optimization. It signals cells using a combination of three major communication planes (biochemical composition, biomechanical properties and topography). This complexity in communication allows the ECM to orchestrate processes such as tissue healing and regeneration.
By utilizing the biophysical principle of MacroMolecular Crowding (MMC) they can generate cell-derived ECMs in vitro in sufficient amounts and with a stable and specified bioactivity1, 2. This emulation of crowded conditions, as found in the physiological extracellular space, results in increased thermodynamic activity and reaction kinetics in the culture system1. They have shown previously that MMC augmented ECM assembly3 and deposition1, 2, ensuring strong and stable bioactivity, far exceeding the abilities of an ECM generated under uncrowded conditions2. Current focus lies of the engineering of matrices, which will improve pathological chronic inflammatory tissue environments.

Figure 1: MMC drives cell-specific ECM deposition. HSPG = heparan sulfate proteoglycan
Selected Publications
- Chen C, Loe F, Blocki, A, Peng Y, Raghunath M. “Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies.” Adv Drug Deliv Rev, 2011; 63(4-5):277–290.
- Ang XM, Lee MHC, Blocki, A, Chen C, Ong LLS, Asada HH, Sheppard A, Raghunath M. “Macromolecular crowding amplifies adipogenesis of human bone marrow-derived mesenchymal stem cells by enhancing the pro-adipogenic microenvironment.” Tissue Eng Part A, 2014; 20(5-6):966–981.
- Dewavrin JY, Abdurrahiem M, Blocki, A, Musib M, Piazza F, Raghunath M. “Synergistic rate boosting of collagen fibrillogenesis in heterogeneous mixtures of crowding agents.” J Phys Chem B, 2015; 119(12):4350–4358.
Blood-derived angiogenic cells (BDACs) with a pericytic phenotype for the treatment of ischemic diseases
Using MMC we were able to source functional cells in clinically relevant numbers and time frame from an easy accessible cell source, namely peripheral blood. These BDACs were demonstrated to promote angiogenesis in vitro and in vivo and even rescue the affected limb in a murine pre-clinical model of critical limb ischemia (CLI)6.
By using BDACs, many limitations of current autologous cell-based therapies can be addressed. Hence, we are further exploring the functionality and mechanism of action of BDACs with specific focus on the treatment of ischemic diseases.
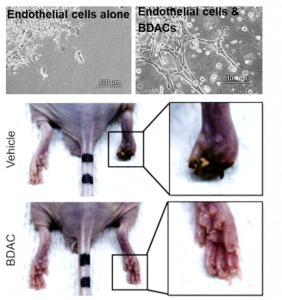
Figure 2: BDACs enhance endothelial sprouting in vitro and revascularization in CLI model in vivo.
Not all pericytes are mesenchymal stem cells (MSCs). Indeed, specifically during angiogenesis pericytes of hematopoietic origin were demonstrated to be the major supportive cell type4, 5.
Selected Publications
- Blocki, A, Beyer S, Jung F, Raghunath M. “The controversial origin of pericytes during angiogenesis – Implications for cell-based therapeutic angiogenesis and cell-based therapies.” Clin Hemorheol Microcirc, 2018; 69(1-2):215–232.
- Blocki, A, Wang Y, Koch M, Peh P, Beyer S, Law P, Hui J, Raghunath M. “Not all MSCs can act as pericytes: functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis.” Stem Cells Dev, 2013; 22(17):2347–2355.
- Blocki, A, Wang Y, Koch M, Goralczyk A, Beyer S, Agarwal N, Lee M, Moonshi S, Dewavrin J-Y, Peh P, Schwarz H, Bhakoo K, Raghunath M. “Sourcing of an alternative pericyte-like cell type from peripheral blood in clinically relevant numbers for therapeutic angiogenic applications.” Mol Ther, 2015; 23(2):510–522.
Fine-tuning three-dimensional microenvironments for tissue engineering
ECM-derived proteins and carbohydrates are advantageous biopolymers for engineering of biocompatible and cell-supportive hydrogels. By fine-tuning mechanical properties and biochemical compositions these hydrogels can be utilized as a simplified version of cellular microenvironments for tissue engineering7–9. Her current focus lies on hydrogels that support microvasculature formation, stabilization and functionality for micro-physiological tissue engineering.

Figure 3: Formation of microvascular networks in gelatin-based hydrogels.
Selected Publications
- Assunção, M., Dehghan-Baniani, D., Yiu, C.H.K., Später, T., Beyer, S. Blocki, A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine, Front. Bioeng. Biotechnol. 8 (2020) 602009. doi:10.3389/fbioe.2020.602009.
- Assunção, M. , Yiu, C.H.K., Wan, H.-Y., Wang, D., Ker, D.F.E., Tuan, R.S., Blocki, A. Hyaluronic acid drives mesenchymal stromal cell-derived extracellular matrix assembly by promoting fibronectin fibrillogenesis, J. Mater. Chem. B. 9 (2021) 7205–7215. doi:10.1039/d1tb00268f.
- Wan, H.-Y.#, SHIN, R.L.Y.#, Chen ,J.C.H., Assunção, M., Wang, D., Nilsson, S.K., Tuan, R.S., Blocki, A. Dextran Sulfate-amplified Extracellular Matrix Deposition Promotes Osteogenic Differentiation of Mesenchymal Stem Cells, Acta Biomater. (2021). doi:10.1016/J.ACTBIO.2021.11.049. (# Shared first authorship)
- Chiang, C.E., Fang, Y.Q., Ho, C.T., Assunção, M., Lin, S.J., Wang,Y.C., Blocki, A.*, Huang, C.C.*. Bioactive Decellularized Extracellular Matrix Derived from 3D Stem Cell Spheroids under Macromolecular Crowding Serves as a Scaffold for Tissue Engineering, Adv. Healthc. Mater. 10 (2021) 2100024. doi:10.1002/adhm.202100024. (* shared corresponding authorship)
- Blocki, A, Löwenberg C, Jiang Y, Kratz K, Neffe AT, Jung F, Lendlein A. “Response of encapsulated cells to a gelatin matrix with varied bulk and microenvironmental elastic properties.” Polym Adv Technol, 2017; 28:1245–1251.
- Blocki, A, Löper F, Chirico N, Neffe AT, Jung F, Stamm C, Lendlein A. “Engineering of cell-laden gelatin-based microgels for cell delivery and immobilization in regenerative therapies.” Clin Hemorheol Microcirc, 2017; 67(3-4):251–259.
- Blocki, A, Beyer S, Dewavrin JY, Goralczyk A, Wang Y, Peh P, Ng M, Moonshi SS, Vuddagiri S, Raghunath M, Martinez EC, Bhakoo KK. “Microcapsules engineered to support mesenchymal stem cell (MSC) survival and proliferation enable long-term retention of MSCs in infarcted myocardium.” Biomaterials, 2015; 53:12–24.
Grant Record
- ITSP Seed Project [PI; 01-Aug-21 to 31-Dec-22]: “Development of a Nature-Inspired Material for the Treatment of Non-Healing Wounds” (HK$1,316,870).
- HMRF Grant: “Exploring human blood-derived angiogenic cells (BDACs) with pericyte characteristics for the treatment of diabetic chronic wounds.”
(HK$ 990,920) - Direct grant (CUHK Research Committee) [PI; 30-Jun-20 to 29-Jun-21]: “Development of a cell-derived extracellular matrix-based biomaterial for the treatment of impaired bone healing” (HK$51,000).
- ITSP Seed Project [PI; 01-Mar-20 to 31-Aug-21]: “Xeno-Free Sourcing And Delivery Using Microbeads Of Reparative Cells From The Patient’s Own Blood For The Treatment of Critical Limb Ischemia” (HK$1,382,187).
- ITF – Postdoctoral Hub for ITF projects [PI; 01-Mar-20 to 31-Aug-21]: “Xeno-Free Sourcing And Delivery Using Microbeads Of Reparative Cells From The Patient’s Own Blood For The Treatment of Critical Limb Ischemia” (HK$601,967).
- ITF – Research Talent Hub for ITF projects [PI; 05-Oct-20 to 31-Aug-21]: “Xeno-Free Sourcing And Delivery Using Microbeads Of Reparative Cells From The Patient’s Own Blood For The Treatment of Critical Limb Ischemia” (HK$364,264).
- RGC Germany/Hong Kong Joint Research Scheme 2019/20 [PI; 01-Jan-20 to 31-Dec-21]: “Characterization of extracellular matrix (ECM)-based biomaterials with tailored bioactivities by Raman microscopy” (HK$57,400).
- Shun Hing Institute of Advanced Engineering (SHIAE) grant [PI; 01-Sep-20 to 31-Aug-22]: “Development of a cell-derived extracellular matrix-based biomaterial for the treatment of osteoarthritis” (HK$694,000).
- Postdoctoral fellowship, Charité – Universitätsmedizin zu Berlin, Berlin-Brandenburg School for Regenerative Therapies (BSRT) including 20,000 Euro research grant.

