
Assistant Professor of the Institute for Tissue Engineering and Regenerative Medicine
Office: (852) 3943 3032
Email: honfaichan@cuhk.edu.hk
Address: Room 423A, 4/F, Lo Kwee-Seong Integrated Biomedical Science Building, Area 39, CUHK
ORCID: 0000-0003-0573-1251
SBS Website: https://www2.sbs.cuhk.edu.hk/en-gb/people/academic-staff/prof-chan-hon-fai-vivas
Website: https://honfaichan.wixsite.com/chanlab-cuhk
Personal Statement
Prof. Chan’s long-term research goal is to understand cell-cell and cell-extracellular matrix interactions in human cellular microenvironment and to develop micro- and nano- materials and process to recapitulate the microenvironment for tissue engineering and regenerative medicine.
Contributions to Science and Selected Publications
A. Microscale tissue engineering. Prof. Chan has developed various microfluidic platforms to recapitulate cell-cell and cell-extracellular matrix interactions for tissue engineering.
- Chan HF, Ma S, Leong KW. “Can microfluidics address biomanufacturing challenges in drug/gene/cell therapies?” Regen Biomater, 2016; 3(2):87-98.
- Chan HF, Zhang Y, Leong KW. “Efficient one-step production of microencapsulated hepatocyte spheroids with enhanced functions.” Small, 2016; 12(20):2720-2730.
- Chan HF, Zhang Y, Ho YP, Chiu YL, Jung Y, Leong KW. “Rapid formation of multicellular spheroids in double-emulsion droplets with controllable microenvironment.” Sci Rep, 2013; 3:3462.
B. Engineering hydrogel for biomedical applications. Prof. Chan has developed various mechanically-robust hydrogels to overcome the challenge of poor mechanical property when applying hydrogel in biomedicine.
- Liu X, Steiger C, Lin S, Parada GA, Liu J, Chan HF, Yuk H, Phan NV, Collins J, Tamang S, Traverso G, Zhao X. “Ingestible hydrogel device.” Nat Commun, 2019; 10(1):493.
- Chan HF, Zhao R, Parada GA, Meng H, Leong KW, Griffith LG, Zhao X. “Folding artificial mucosa with cell-laden hydrogels guided by mechanics models.” Proc Natl Acad Sci U S A, 2018; 115(29):7503-7508.
- Hong S*, Sycks D*, Chan HF*, Lin S, Lopez GP, Guilak F, Leong KW, Zhao X. “3D printing of highly stretchable and tough hydrogels into complex, cellularized structures.” Adv Mater, 2015; 27(27):4035-4040.
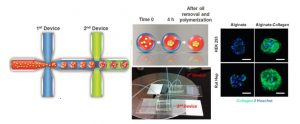
Hepatocyte spheroids can contribute to liver research in various capacities. However, the technique of spheroid culture is far from optimal, as it is challenging to generate and maintain uniform-sized spheroids on a large scale. Moreover, a lack of control of cell-ECM interaction is often seen in spheroid cultures because no exogenous ECM is supplied. Here, a microfluidics technology has been developed to generate uniform-sized hepatocyte spheroids followed by encapsulation into microscale alginate hydrogel (microgel). The function of hepatocyte spheroid could be enhanced by premixing exogenous ECM cues (collagen type I) with cells before injecting into the platform.
They demonstrated improved hepatocyte functions during 24 d culture (albumin secretion, urea secretion, and cytochrome P450 activity) when hepatocyte spheroid was encapsulated in alginate-collagen microgel as compared to alginate alone and 2D collagen sandwich culture. This microencapsulated-spheroid formation technology with high yield, versatility, and uniformity is envisioned to be an enabling technology for liver tissue engineering as well as biomanufacturing. (H.F. Chan et al. Small 2016)
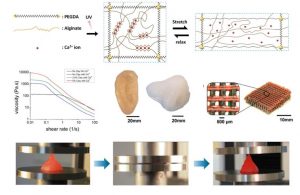
Living tissues such as cartilage usually have high fracture toughness in order to withstand substantial internal and external mechanical loads. The high toughness of tissues challenges researchers to design hydrogels capable of achieving similar toughness in order to withstand physiological mechanical loads. Despite recent success in developing tough hydrogels, the fabrication of these hydrogels often involves toxic chemicals and/or harsh reactions, limiting their capability to encapsulate cells. In addition, it is desirable to fabricate cell-embedded hydrogels with macroporous architecture conducive to generation of complex tissues. While 3D printing offers rapid prototyping and can print hydrogels into complex 3D structures for functions such as vascular networks and aortic valves, it has not been possible to print tough hydrogels into complex structures other than simple and flat ones such as dog-bone samples. Here, they chose the biocompatible materials sodium alginate and poly (ethylene glycol) (PEG) to constitute an interpenetrating network. The resultant hydrogel of covalently crosslinked PEG and ionically crosslinked alginate possesses high fracture toughness and allows cell encapsulation. They also demonstrate the capability of printing the PEG–alginate hydrogels into various complicated 3D structures by controlling the viscosity of the pregel solution using a biocompatible nanoclay material. The 3D printed structure can withstand a high compressive strain (95%) without collapsing and return to its original shape after relaxation. (S. Hong*, D. Sycks*, H.F. Chan* et al. Advanced Materials 2015)
Grant Record
- National Key Research and Development Program of China 2019 [Co-PI; 01-Dec-19 to 30-Nov-23]: “Smart biomaterials for directing stem cell differentiation in 3D for constructing bioartificial liver” (RMB$1,660,000).
- National Key Research and Development Program of China 2018 [Co-PI; 01-Jul-19 to 30-Jun-24]: “Engineering microbial chassis for synthetic biology applications” (RMB$1,270,000).
- RGC – General Research Fund [PI; 01-Jan-20]: “Development of a microfluidic platform for biofabricating 3D spheroid entrapped in combinatorial microenvironment for optimized hepatocyte culture” (HK$343,034).
- Shun Hing Institute of Advanced Engineering Project Fund [PI; 01-Jul-19]: “Development of a bilayer folded scaffold for intestinal tissue engineering” (HK$800,000).

