
Research Assistant Professor of the Institute for Tissue Engineering and Regenerative Medicine
Office: (852) 3943 0645
Email: chienweilee@cuhk.edu.hk
Address: Room 107A, 1/F, Lo Kwee-Seong Integrated Biomedical Science Building, Area 39, CUHK
ORCID: 0000-0002-6679-0020
SBS Website: https://www2.sbs.cuhk.edu.hk/en-gb/people/academic-staff/prof-lee-chien-wei
Personal Statement
Mesenchymal stem cells (MSCs) hold great promise in tissue regeneration and ageing-related problems through the paracrine effects and multipotent differentiation potential. Prof. Lee has demonstrated that MSC and MSC-derived products reduce metabolic syndrome through paracrine effects. Besides, epigenetically manipulation in MSCs accelerates cell fate switch to facilitate the accessibility of cell transplantation. Now, the primary research theme is to develop a disease-in-a-dish model for liver disease and explore the therapeutic potential and the mechanisms of MSCs and their exosomes in liver disease.
Research Interests
- Exploration of the lineage plasticity of MSCs in hepatogenic differentiation
- Study the application of MSC-derived exosomes in liver diseases
- Investigation of the use of stem cell-based therapy to treat ageing-related problems
Selected Publications
A. Mesenchymal stem cell-based therapy in liver diseases
- Lee CW#, Chen YF#, Wu HH#, Lee OK. “Historical perspectives and advances in Mesenchymal Stem Cell Research for the treatment of liver diseases.” Gastroenterology, 2018; 154(1):46-56. (#These authors contributed equally to this manuscript)
- Lee CW, Huang CW, Huang HD, Huang YH, Ho JH, Yang MH, Yang VW, Lee OK. “DNA methyltransferases modulate hepatogenic lineage plasticity of mesenchymal stromal cells.” Stem Cell Reports, 2017; 9(1):247-263.
- Lee CW, Hsiao WT, Lee OK. “Mesenchymal stromal cell-based therapies reduce obesity and metabolic syndromes induced by a high-fat diet.” Transl Res, 2017; 182:61-74.e8.
B. Mechanistic study of osteogenesis in MSCs and osteoporosis
MSC-based therapies are able to reduce nonalcoholic fatty liver disease
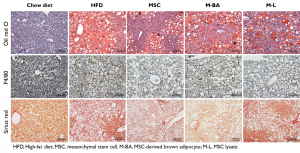
Obesity is an alarming global health problem that results in multiaspect metabolic syndromes in both genders and most age groups. The lack of effective therapies for obesity and its associated metabolic syndrome is an urgent societal issue. They found that repeated transplantation of human MSCs, MSC-derived brown adipocytes (M-BA), and MSC lysate into obese mice are able to improve obesity-associated metabolic syndromes including nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, glucose intolerance, and inflammation in obese mice. In addition, MSC-based treatments altered the ratio of adiponectin to leptin to maintaining energy homeostasis, in major metabolic tissues. Together, MSCs and their derivatives have great potential to treat metabolic syndrome. (Lee CW et al. Transl Res. 2017 Apr.)
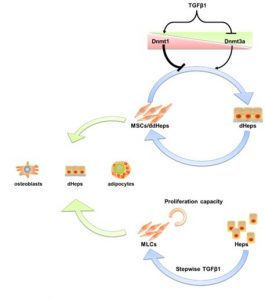
TGFβ1-Dnmts axis control the hepatic plasticity of MSCs
Transl Res. 2017 Apr;182:61-74.e8. Stem Cell Reports. 2017 Jul 11;9(1):247-263
The irreversibility of developmental processes in mammalian cells has been challenged by rising evidence that de-differentiation of hepatocytes occurs in adult liver. However, the underlying mechanism is still unclear. Here they found that the hepatic lineage reversibility is modulated by DNA methyltransferases (DNMTs) through studying within a useful model, hepatogenic differentiation of MSCs. Respectively, DNMT1 repressed and DNMT3a promoted hepatogenic differentiation. Besides, DNMT1 knockdown shortened hepatogenic differentiation time from 28 to 14 days. DNMTs are regulated by TGFb1, which in turn controls hepatogenic differentiation and de-differentiation. Also, a stepwise reduction in TGFb1 concentrations in culture alters the expression of DNMTs in primary hepatocytes (Heps) and confers Heps with multi-differentiation potentials similarly to MSCs. This previously unrecognized TGFb1-DNMTs-MSC-HD axis may further increase the understanding the normal and pathological processes in the liver, as well as functions of MSCs after transplantation to treat liver diseases. (Lee CW, et al. Stem cell reports. 2017 Jun.)
