
Assistant Professor of the School of Biomedical Sciences & the Institute for Tissue Engineering and Regenerative Medicine
Office: (852) 3943 0681
Email: wangmd@cuhk.edu.hk
Address: Room 422A, 4/F, Lo Kwee-Seong Integrated Biomedical Science Building, Area 39, CUHK
ORCID: 0000-0002-5934-9085
SBS Website: https://www2.sbs.cuhk.edu.hk/en-gb/people/academic-staff/prof-wang-dan-michelle
Personal Statement and Research Goals
To augment repair of hard-to-soft, multi-tissue musculoskeletal units such as the bone-tendon interface is challenging. Specifically, precise control of biomechanical and biochemical cues is required to support tissue healing and functional movement. Prof. Wang’s long-term goals are to utilize an interdisciplinary approach, including extracellular matrix (ECM) biology, stem cell biology, biomaterial development, together to achieve a spatially and temporally guided, efficacious bone-tendon tissue regeneration.
Contributions to Science and Selected Publications
A. Extracellular matrix biology and regenerative medicine. Her group has developed a urea-based, non-enzymatic, low-cost approach for preparing soluble ECM extracts from juvenile bovine tendons, which shows strong tenogenic effects on adult stem cells. Her group is currently seeking to understand the mechanisms of actions of tendon ECM bioactivity and identifying the functional components to justify their rational application for tendon tissue engineering.
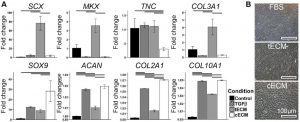
Fig.1. Urea-extracted tissue ECM exhibits tissue-specific bioactivity. (A) Effect of soluble urea extracted ECMs on human mesenchymal stem cells (hMSCs) pellet culture. Gene expression analysis on D21 showed tenogenesis of tendon ECM (tECM) and chondrogenesis of cartilage ECM (cECM). (B) Phase contract microscope showed spindle-shaped cells in tECM-supplemented hMSCs culture group and cobblestone morphology with cECM supplementation. (Tuan, et al., 2017)
B. Biomaterial fabrication and development. With the combined use of macroscale biomechanical (material stiffness) and biochemical cues (growth factor biopatterning), her group has developed a novel, bio-functionalized, mechanically-graded biomaterial that holds promise for hard-to-soft tissue regeneration such as rotator cuff repair.
- Ker DFE*, Wang D*, Behn AW, Wang ETH, Zhang X, Zhou BY, Mercado-Pagan AE, Kim S, Kleimeyer J, Gharaibeh B, Shanjani Y, Nelson D, Safran M, Cheung E, Campbell P, Yang YP. “Functionally-Graded, Bone- and Tendon-Like Polyurethane for Rotator Cuff Repair.” Advanced Functional Materials, 2018; 28(20):1707107. (*Co-first authors)
- Wang D, Xu Y, Lu C, Yang X, Zhang D, Shao L, Wang R. “Comparison of fracture strength and fracture modes of zirconia dental ceramics manufactured by four different CAD/CAM systems.” Key Engineering Materials, 2012; 492:30-34.

Fig. 2. (A) Mechanically-graded biomaterial for bone-tendon repair. (B) Bone-like and (C) tendon-like tissue patterning on mechanically-graded biomaterial in vivo. Tri, trichrome.
C. Immunomodulation for enhanced fracture repair. Her group has studied the role of inflammatory mediators, Toll-like receptors (TLRs) on fracture repair. Biological insights gained from such studies can be utilized to achieve a pro-regenerative host immune response for successful bone repair.
- Wang D, Gilbert JR, Taylor GM, Sodhi CP, Hackam DJ, Losee JE, Billiar TR, Cooper GM. “TLR4 inactivation in myeloid cells accelerates bone healing of a calvarial defect model in mouse.” Plastic and Reconstructive Surgery, 2017; 140(2):296e-306e.
- Wang D, Taylor GM, Gilbert JR, Sodhi CP, Hackam DJ, Losee JE, Billiar TR, Cooper GM. “Enhanced calvarial bone healing in CD-11c-TLR4-/- and Myd88-/- mice.” Plastic and Reconstructive Surgery, 2017; 139(4):933e-940e.
- Wang D, Gilbert JR, Cray JJ Jr, Kubala A, Shaw MA, Billiar TR, Cooper GM. “Toll-like receptor 4 mediates the regenerative effects of bone grafts for calvarial bone repair.” Tissue Engineering Part A, 2015; 21(7-8):1299-1308.
- Shakir S, MacIsaac ZM, Naran S, Smith DM, Cray JJ, Craft TK, Angle S, Clafshenkel W, Shaw MA, Wang D, Weiss LE, Campbell P, Mooney MP, Losee JE, Cooper GM. “Transforming growth factor beta 1 improves bone healing and promotes suture regeneration.” Tissue Engineering Part A, 2015; 21(5-6):939-947.
- Wang D, Gilbert JR, Cray JJ Jr, Kubala A, Shaw MA, Billiar TR, Cooper GM. “Accelerated calvarial healing in mice lacking Toll-like receptor 4.” PLoS One, 2012; 7(10):e46945.
- Ker DFE, Wang D, Sharma S, Zhang B, Passarelli B, Neff N, Li C, Maloney W, Quake S and Yang YP. “Identifying deer antler uhrf1 proliferation and s100a10 mineralization genes using comparative RNA-seq.” Stem Cell Research & Therapy, 2018; 9(1):292.
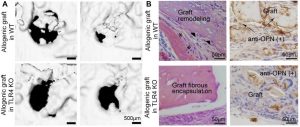
Fig. 3. TLR4 signal is essential for graft-mediated bone repair. Allogeneic grafts from donor WT mice were implanted into skull defects of recipient WT and TLR4 KO mice. (A) Live µCT and (B) histology analyses showed that inhibition of TLR4 in allogeneic grafts hindered graft remodeling and bone healing. (Wang, et al., 2015 and unpublished data)
Grant Record
- The National Science Foundation of China, General program [PI; 2018-2022]: “Investigating the role of HMGB1/TLR4-mediated osteoclastogenesis in allograft remodeling”.
- Innovation Technology Fund Tier 3 Grant [PI]: “Developmentally-inspired biomaterials for tendon repair” (HK$1,308,000).
- NSFC funded grant [PI; 2012-2015]: “Cell necrosis promotes bone regeneration by virtue of an inflammatory response through TLR4 signal pathway”.
