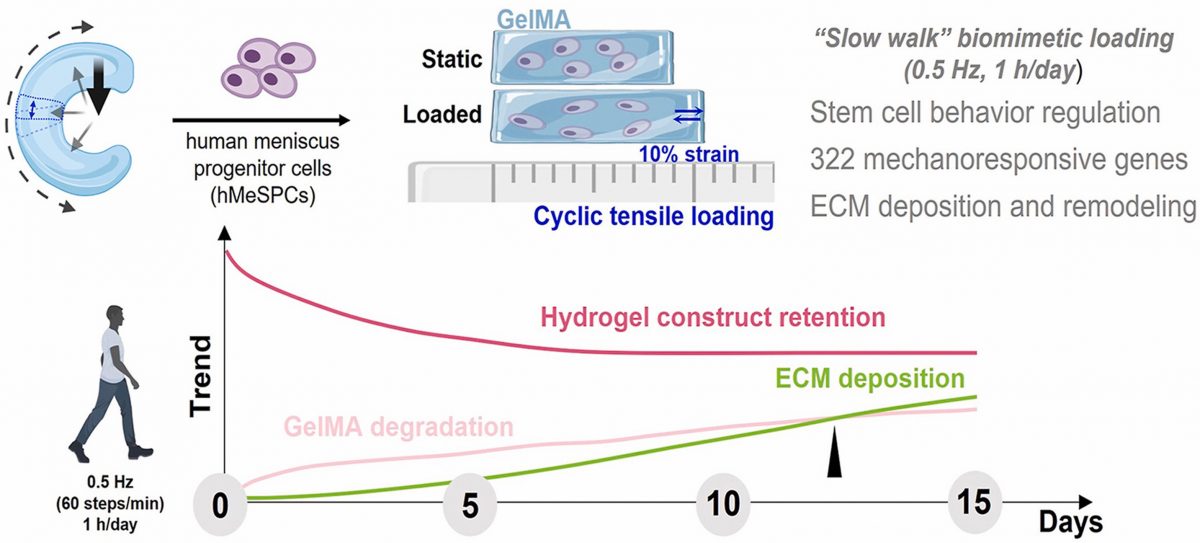
This research project is completed under the supervision of Prof. JIANG Yangzi and Prof. TUAN S. Rocky, and Dr. SUN Jing is the first author. It is worth noting that Dr. SUN Jing, who took part in this project, was an iTERM/SBS Ph.D. student who graduated in 2021 and is now a postdoctoral fellow. https://doi.org/10.1016/j.bioactmat.2023.01.025 Here are some extracts from their work: “It is recently reported that healthy adult takes approximately 6,000–9,000 steps/day, while advancing age or presence of chronic disease/disability generally reduces levels of activity to 1200–2000 steps/day; whether such low activity is sufficient for joint tissue health is currently unknown. The mechanical loading regimen adopted here (10% strain, 0.5 Hz, 1 h/day) was selected to mimic cyclic tensile loading of the meniscus in a “slow walk”, in terms of strain, frequency and a low level of daily activity (1,800 loads/knee/day, i.e., around 1/2 of average number of steps/day). The observed effects of this biomimetic mechanical loading on the activities and behavior of hMeSPCs in 3D GelMA hydrogel constructs support the significant contribution of mechanical factors in meniscus tissue maintenance. Experimentally, a “slow walk” biomimetic cyclic loading regimen (10% tensile strain, 0.5 Hz, 1 h/day, up to 15 days) is applied to hMeSPCs encapsulated in GelMA hydrogel with a magnetic force-controlled loading actuator. The loading significantly increases cell differentiation and fibrocartilage-like ECM deposition without affecting cell viability. Transcriptomic analysis reveals 332 mechanoresponsive genes, clustered into cell senescence, mechanical sensitivity, and ECM dynamics, associated with interleukins, integrins, and collagens/matrix metalloproteinase pathways. The cell-GelMA constructs show active ECM remodeling, traced using a green fluorescence tagged (GFT)-GelMA hydrogel. Loading enhances nascent pericellular matrix production by the encapsulated hMeSPCs, which gradually compensates for the hydrogel loss in the cultures. These findings demonstrate the strong tissue-forming ability of hMeSPCs, and the importance of mechanical factors in maintaining meniscus homeostasis. It is noteworthy that, different from previous studies, the serum-free, TGF-β-free culture condition used here minimized potential effects of FGF2 and TGF-βs present in serum on stem cell regulation. In summary, we have developed a novel platform to examine how biomimetic loading regulates a mechano-responsive resident stem/ progenitor cell population derived from human meniscus. These in vitro findings provide insights on the mechanobiology of meniscal tissue, and strongly suggest that, in vivo, a mild activity level similar to that of a slow walk would be beneficial in the maintenance of a cartilage phenotype in the meniscus, in formation that is relevant for the optimization of meniscus tissue engineering and regeneration.”
Acknowledgements This work was supported by the National Key R&D Program [grant number 2019YFA0111900, to YJ], administered by the Ministry of Science and Technology of the People’s Republic of China (MOST, China); General Research Fund (GRF, grant number 14104022, to YJ) by Hong Kong Research Grants Council, University Grants Committee (RGC, UGC) of Hong Kong SAR, China; The Chinese University of Hong Kong, Impact Postdoctoral Fellowship Scheme [IPDFS, CUHK, to JS]; the Center for Neuromusculoskeletal Restorative Medicine [CNRM at InnoHK, to RST, YJ] by Innovation and Technology Commission (ITC) of Hong Kong SAR, China; and Lee Quo Wei and Lee Yick Hoi Lun Professorship in Tissue Engineering Regenerative Medicine of The Chinese University of Hong Kong (to RST). The authors thank the CORE Facilities of School of Biomedical Sciences, CUHK. Article information Jing Sun, Yau Tsz Chan, Ki Wai Kevin Ho, Li Zhang, Liming Bian, Rocky S. Tuan*, Yangzi Jiang*. “Slow walk” mimetic tensile loading maintains human meniscus tissue resident progenitor cells homeostasis in photocrosslinked gelatin hydrogel. Bioactive Materials, 25, (2023) 256-272. DOI: 10.1016/j.bioactmat.2023.01.025






Leave A Comment