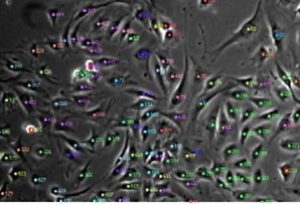
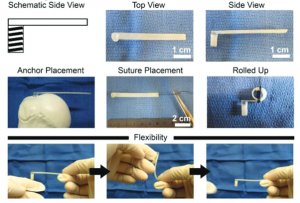
Label-free cell analysis is a promising technology that is broadly applicable to the biomedical sciences but cannot be easily accomplished without artificial intelligence. Simply put, it is very tedious and time consuming for research scientists to manually trace the outlines of cells for thousands of images in an accurate and reproducible manner so as to quantify cell population metrics and derive biological insights. The capability to continuously monitor cell populations at the resolution of single cells without needing to add external labelling reagents that may alter cell behaviour or require endpoint measurements would not only facilitate cost-effective research but also allow researchers to answer previously unanswered basic scientific questions on stem cell lineage and cell heterogeneity as well as apply such knowledge for translational applications such as stem cell manufacturing. In the Ker Lab, we are working on computer vision technologies that aim to achieve universal cell detection and tracking of mammalian cells. Our work includes generation of curated microscopy datasets including the world’s largest annotations for human- and computer-tracked cells (https://doi.org/10.1038/sdata.2018.237; https://doi.org/10.17605/OSF.IO/YSAQ2; 10,384,828 computer-detected cells and 1,015,195 human-annotated cells) and computer vision-aided cell manufacturing (https://doi.org/10.1371/journal.pone.0027672). Image of tracked mouse muscle progenitor cells. Biomaterials are promising tools for understanding cell interactions with their microenvironment as well as potential therapeutics for injury treatment. Deriving physiologically relevant insights and achieving practical therapeutic implementation requires detailed understanding of cellular responses to biomaterials. In the Ker Lab, we employ a mix of in silico, ex vivo, in vitro, and in vivo approaches to develop different biomaterials aimed at mimicking native tissues and engineer desired biomaterial traits in order to better understand cell and biomaterial behaviours. These approaches include finite element analysis, polymer synthesis and 3D-printing, chemical and mechanical characterization, cell-based assays, and animal modelling. Our recent work includes characterizing the combined interaction of biomaterial stiffness and growth factors on musculoskeletal cell differentiation (https://doi.org/10.1038/s41427-021-00294-z) and development of a hard-to-soft material for bone-tendon repair (https://doi.org/10.1002/adfm.201707107). Image of stress-reducing bone-tendon biomaterial.






Leave A Comment