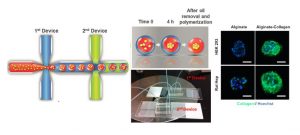
Hepatocyte spheroids can contribute to liver research in various capacities. However, the technique of spheroid culture is far from optimal, as it is challenging to generate and maintain uniform-sized spheroids on a large scale. Moreover, a lack of control of cell-ECM interaction is often seen in spheroid cultures because no exogenous ECM is supplied. Here, a microfluidics technology has been developed to generate uniform-sized hepatocyte spheroids followed by encapsulation into microscale alginate hydrogel (microgel). The function of hepatocyte spheroid could be enhanced by premixing exogenous ECM cues (collagen type I) with cells before injecting into the platform.
We demonstrated improved hepatocyte functions during 24 d culture (albumin secretion, urea secretion, and cytochrome P450 activity) when hepatocyte spheroid was encapsulated in alginate-collagen microgel as compared to alginate alone and 2D collagen sandwich culture. This microencapsulated-spheroid formation technology with high yield, versatility, and uniformity is envisioned to be an enabling technology for liver tissue engineering as well as biomanufacturing. (H.F. Chan et al. Small 2016)

Living tissues such as cartilage usually have high fracture toughness in order to withstand substantial internal and external mechanical loads. The high toughness of tissues challenges researchers to design hydrogels capable of achieving similar toughness in order to withstand physiological mechanical loads. Despite recent success in developing tough hydrogels, the fabrication of these hydrogels often involves toxic chemicals and/or harsh reactions, limiting their capability to encapsulate cells. In addition, it is desirable to fabricate cell-embedded hydrogels with macroporous architecture conducive to generation of complex tissues. While 3D printing offers rapid prototyping and can print hydrogels into complex 3D structures for functions such as vascular networks and aortic valves, it has not been possible to print tough hydrogels into complex structures other than simple and flat ones such as dog-bone samples. Here, we chose the biocompatible materials sodium alginate and poly (ethylene glycol) (PEG) to constitute an interpenetrating network. The resultant hydrogel of covalently crosslinked PEG and ionically crosslinked alginate possesses high fracture toughness and allows cell encapsulation. We also demonstrate the capability of printing the PEG–alginate hydrogels into various complicated 3D structures by controlling the viscosity of the pregel solution using a biocompatible nanoclay material. The 3D printed structure can withstand a high compressive strain (95%) without collapsing and return to its original shape after relaxation. (S. Hong*, D. Sycks*, H.F. Chan* et al. Advanced Materials 2015)






Leave A Comment